CLASSIFICATION AND NOMENCLATURE OF ENZYMES
● Thousands of `color{violet}("enzymes")` have been discovered, isolated and studied.
● Most of these `color{violet}("enzymes")` have been classified into different groups based on the type of reactions they `color{violet}("catalyse.")`
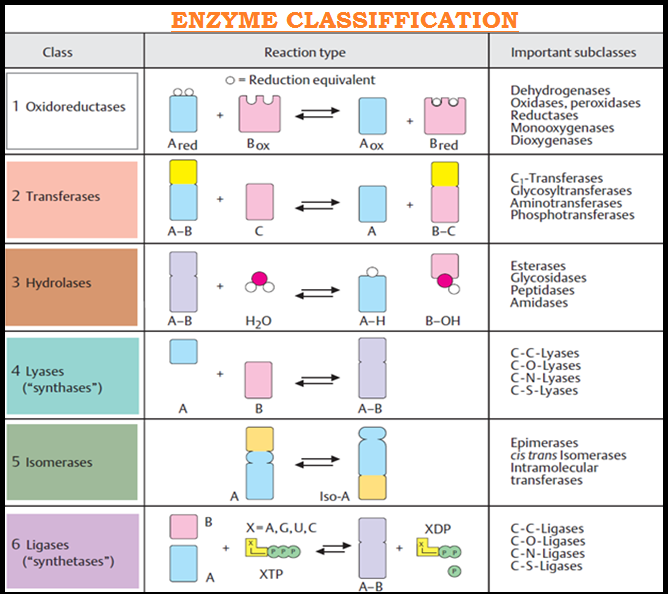
● `color{violet}("Enzymes")` are divided into `color{brown}("6 classes")` each with `color{brown}("4-13 subclasses")` and named accordingly by a `color{brown}("four-digit number.")`
`color{green}(star "Oxidoreductases/dehydrogenases:")` Enzymes which `color{violet}("catalyse oxidoreduction")` between two substrates `S` and `S’` e.g.
`color{violet}" S reduced + S’ oxidised →S oxidised + S’ reduced.")`
`color{green}(star "Transferases:")` Enzymes catalysing a transfer of a group, `color{brown}(G)` (other than hydrogen) between a pair of substrate `S` and `S’` e.g.
`color{violet}("S - G + S’ → S + S’ - G)`
`color{green}(star "Hydrolases:")` Enzymes catalysing hydrolysis of ester, ether, peptide, `color{violet}("glycosidic, C-C, C-halide or P-N bonds")`.
`color{green}(star "Lyases:")` Enzymes that `color{violet}("catalyse removal")` of groups from substrates by `color{violet}("mechanisms")` other than `color{violet}("hydrolysis leaving double bonds.")`
`color{violet}(overset(overset(X)(|))C - overset(overset(Y)(|))C → X-Y + C = C)`
`color{green}(star "Isomerases:")` Includes all `color{violet}("enzymes catalysing inter-conversion")` of optical, geometric or positional isomers.
`color{green}(star "Ligases:")` Enzymes catalysing the linking together of 2 compounds, e.g., enzymes which `color{violet}("catalyse")` joining of `color{violet}("C-O, C-S, C-N, P-O")` etc. bonds.
● Most of these `color{violet}("enzymes")` have been classified into different groups based on the type of reactions they `color{violet}("catalyse.")`
● `color{violet}("Enzymes")` are divided into `color{brown}("6 classes")` each with `color{brown}("4-13 subclasses")` and named accordingly by a `color{brown}("four-digit number.")`
`color{green}(star "Oxidoreductases/dehydrogenases:")` Enzymes which `color{violet}("catalyse oxidoreduction")` between two substrates `S` and `S’` e.g.
`color{violet}" S reduced + S’ oxidised →S oxidised + S’ reduced.")`
`color{green}(star "Transferases:")` Enzymes catalysing a transfer of a group, `color{brown}(G)` (other than hydrogen) between a pair of substrate `S` and `S’` e.g.
`color{violet}("S - G + S’ → S + S’ - G)`
`color{green}(star "Hydrolases:")` Enzymes catalysing hydrolysis of ester, ether, peptide, `color{violet}("glycosidic, C-C, C-halide or P-N bonds")`.
`color{green}(star "Lyases:")` Enzymes that `color{violet}("catalyse removal")` of groups from substrates by `color{violet}("mechanisms")` other than `color{violet}("hydrolysis leaving double bonds.")`
`color{violet}(overset(overset(X)(|))C - overset(overset(Y)(|))C → X-Y + C = C)`
`color{green}(star "Isomerases:")` Includes all `color{violet}("enzymes catalysing inter-conversion")` of optical, geometric or positional isomers.
`color{green}(star "Ligases:")` Enzymes catalysing the linking together of 2 compounds, e.g., enzymes which `color{violet}("catalyse")` joining of `color{violet}("C-O, C-S, C-N, P-O")` etc. bonds.
